PRODUCT IDENTIFICATION
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
off-white corrosive crystals
APPLICATIONS
Methylsulphonic acid has applications in the fields include:
- Electroplating and electrochemical processes
- Alkylation, esterification and condensation reactions
- Lead recovery
- Chemical intermediate for manufacture of drugs and pesticides
- Petroleum refining and lube oil additives
- Extraction and process solvent
- Plastic stabilizers
Mesyl Chloride is widely used as a catalyst, chlorination agent and stabilizer for organic synthesis and as an intermediate for photographic chemicals, agrochemcials, dyses and pharmaceuticals. It can be used as a bactericide.
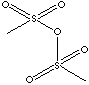
Anhydride is a chemical compound formed by the abstraction of a molecule of water, H2O, from a substance. The term acid anhydride is restricted sometime to the anhydride formed especially from an acid by dehydration or one that revert to the original substance upon hydration. In case of bimolecular, it can be composed of two molecules of the corresponding acid. The term mixed anhydride is an acid anhydride composed of two different acids. Examples are adenosine triphosphate or an aminoacyl adenylate. The anhydrides of bases are oxides. Anhydrides of inorganic acids are usually oxides of nonmetallic elements. Carbon dioxide (CO2) is the anhydride of carbonic acid, dinitrogen pentoxide (N2O5) is the anhydride of nitric acid, and, phosphorus pentoxide (P2O5) is the anhydride of phosphoric acid, and sulfur trioxide (SO3) is the anhydride of sulfuric acid. Examples of inorganic anhydrides include dinitrogen pentoxide, which is the anhydride of nitric acid, and sulfur trioxide, which is the anhydride of sulfuric acid. Organic anhydrides contain the carbonyl group (CO). Organic anhydrides are formed by the condensation of original acids. Lactone, an internal cyclic monoester, is an anhydride derived from the hydroxyl and carboxyl radicals. The names of anhydrides derived from carboxylic acids are given first from the original acid followed by the separate word anhydride. Anhydrides are more reactive than the parent acids. Anhydrides are typically not target molecules, but rather they are used as intermediates for the synthesis of other organic members such as esters and amides for the industrial applications include dyes, pharmaceuticals, pesticides, plastics, fibers, curing agents, plasticizers and many others. Methanesulfonic anhydride is useful in the reaction for aromatic alkylation.
APPEARANCE
off-white crystals
98.0% min
MELTING POINT